A device is in class II if general controls alone are insufficient to provide reasonable assurance of its safety and effectiveness and there is sufficient information to establish special controls including the promulgation of performance standards postmarket surveillance patient registries development and. As simple as a tongue depressor As complex as.

Market Access For Medical Software In The United States Vde Medical Devices And Software
Determining Class IV devices by the Classification Rules Class IV medical devices could be determined by the classification rules 6 7 8 13.
Fda class ii medical device definition. Whereas a Class 1 device must only be listed with the FDA and a Class II device must be cleared by the FDA a Class III device must be approved by the FDA. Class I includes devices with the lowest risk and Class III includes those with the greatest risk. The FDA keeps a watchful eye on the medical device industry To win approval for a class III device.
Class I Devices. The Supreme Courts opportunity to define the ill-defined In 2011 the FDA backtracked on its decision. Class I devices are considered to be at the lowest level of risk of all medical devices and are therefore required to comply with the lowest level of regulatory control.
2 Class II means the class of devices that is or eventually will be subject to special controls. Examples of Class I devices include. For more information please visit the Classify Your Medical Device page.
Elastic bandages dental floss and enemas. Please refer to the article Classification Rules for Medical Devices for details. Medical Device Classes Class I General Controls Most exempt from premarket submission Class II Special Controls Premarket Notification 510k.
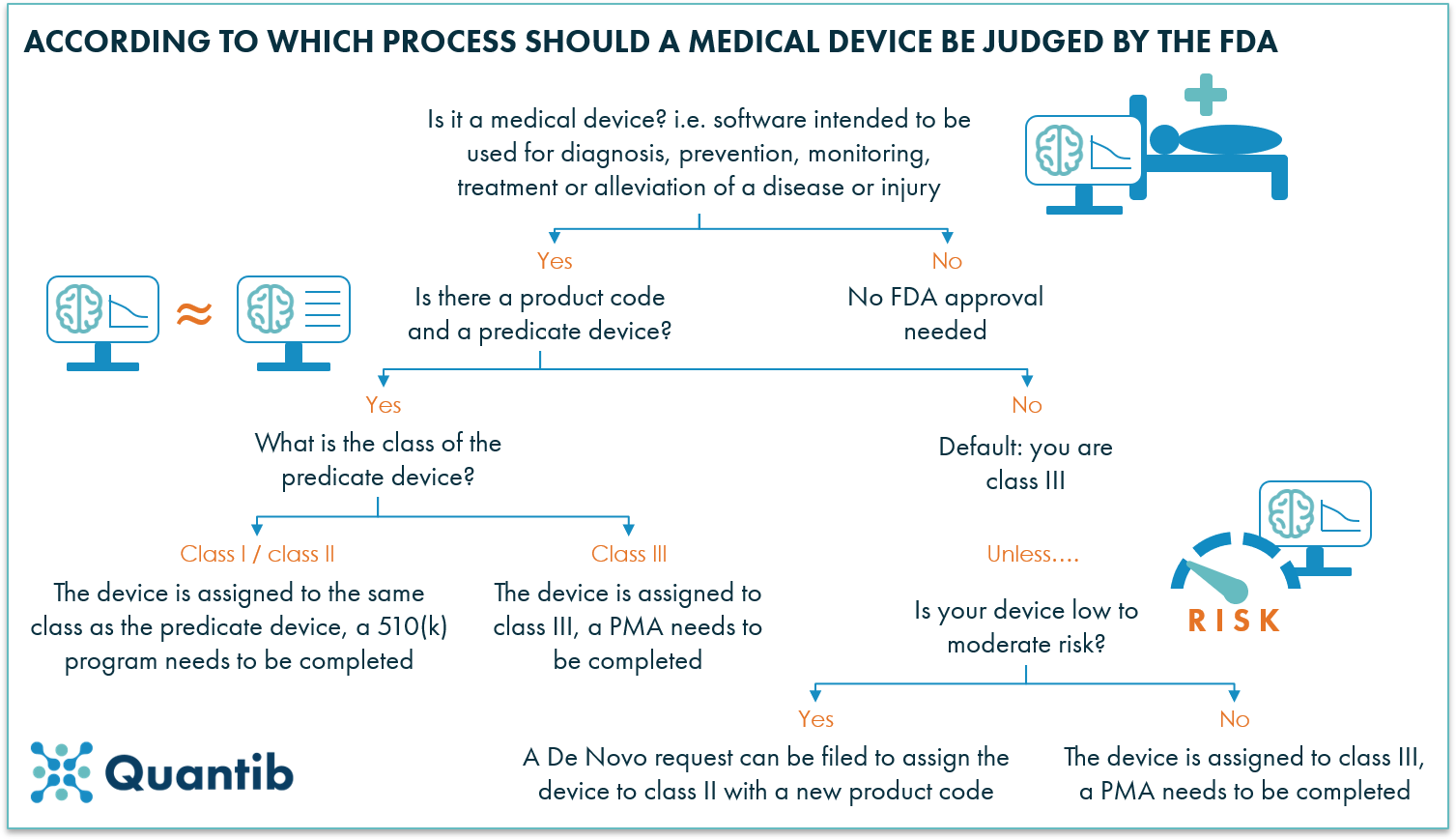
The class to which your device is assigned determines among other things the type of premarketing submissionapplication required for FDA clearance to market. Diagnostic medical devices are classified into four categories Classes I to IV according to their risk levels Class IV being the category of the highest risk and Class I the lowest. Class I devices generally pose the lowest risk.
In some circumstances. Class II devices are defined as those which cannot be classified as class I because the general controls by themselves are insufficient to provide reasonable assurance of safety and effectiveness of the device14 Class II devices can only be marketed after providing the FDA with a pre-market notification also called a 510k. The FDA generally classifies medical devices based on the risks associated with the device and by evaluating the amount of regulation that provides a reasonable assurance of the devices safety and effectiveness.
Class I or Class II device and the device is 1 purported or The contours of the parallel claim exception. Medical device as any product that does not achieve its purposes by chemical action or metabolization. The FDA categorizes medical devices into one of three classes Class I II or III based on their risks and the regulatory controls necessary to provide a reasonable assurance of safety and.

Software As Medical Device Samd Classification And Definitions
Medical Devices Classification As Per Fda Medical Device Regulations Medicaldevices Fda Youtube

A 101 Guide To The Fda Regulatory Process For Ai Radiology Software

Market Access For Medical Software In The United States Vde Medical Devices And Software
